 Bishop Hill
Bishop Hill Phytoplankton love carbon dioxide
 Dec 1, 2015
Dec 1, 2015  Climate: Oceans
Climate: Oceans  Climate: carbon budget
Climate: carbon budget 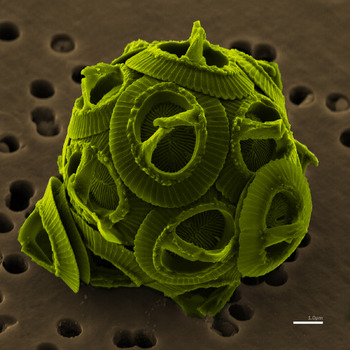 Further to the last post, and with truly magnificent timing, I come across a new paper from John Hopkins University:
Further to the last post, and with truly magnificent timing, I come across a new paper from John Hopkins University:
As anthropogenic CO2 emissions acidify the oceans, calcifiers generally are expected to be negatively affected. However, using data from the Continuous Plankton Recorder, we show that coccolithophore occurrence in the North Atlantic increased from ~2 to over 20% from 1965 through 2010. We used Random Forest models to examine >20 possible environmental drivers of this change, finding that CO2 and the Atlantic Multidecadal Oscillation were the best predictors, leading us to hypothesize that higher CO2 levels might be encouraging growth. A compilation of 41 independent laboratory studies supports our hypothesis. Our study shows a long-term basin-scale increase in coccolithophores and suggests that increasing CO2 and temperature have accelerated the growth of a phytoplankton group that is important for carbon cycling.
So the population of coccolithophores has increased by an order of magnitude. And since coccolithophores sequester carbon dioxide when they calcify, that means a favourable carbon cycle feedback just got a whole lot bigger.
Excellent news, I'm sure you'll agree.



Reader Comments (46)
Finally, some biologists and oceanographers who have heard of the Cretaceous...
The Chalk is basically formed of coccoliths, and was laid down in a massive sedimentary basin extending from the UK across to eastern Europe (Poland). They formed during a time when atmospheric CO2 was roughly 4000 ppm, so an order of magnitude higher than present. No evidence that 'ocean acidification' was sufficient to cause difficulties for these (and lots of other) organisms to precipitate calcite exoskeletons.
Yeah, this has been all over the BBC this morning. Big piece on the Today programme. Not.
That said, they did manage to find a few minutes for Matt Ridley right at the end of the programme. Actually quite a good interview I thought. I'm sure Ward is already complaining.
Another insight into the 'shell game'!
Don't these pesky varmints also increase ocean albedo?
This morning I was googling a story on sea coal harvesting in the Hartlepool Area, the local authority had banned it, ostensibly on health and safety grounds but really to prove their green credentials. Shock horror without people hauling it away every day as they had done for centuries it built up on the beach so they were forced to rescind their ban.
In the process I came across the ultimate green/biofuel marketing lunacy. A UK company called Biomass Fuel Supplies is marketing coal for burning on open fires, one of the most polluting and least efficient methods of heating around that is in fact illegal in most of the UK. It is being sold on the internet under a green banner at over £300 per ton.
I'm slightly puzzled by that use of % of occurrence. Do they mean it occurred in 20% of samples - or that it was 20% of sampled plankton? I can't believe they mean it has a concentration of 20%.
Still, whatever they mean there is obviously a significant increase relative to something!
I assume they mean it is found in 20% of samples instead of about 2% as before.
If the occurrence has increased 10x like this, the population has quite likely increased more than 10x, because not only are there more positive samples, but population levels in the most concentrated samples will probably have increased too.
I read it as from about 2% to 20% increase, which is not the Bish's order of magnitude. So much for precision and clarity in science.
@Keith Willshaw. I'm a bit surprised about burning coal on an open fire being illegal in most of the UK - surely only in so-called smoke control areas? £300 per tonne sounds in line with the local coal merchant's rates here (in Somerset), anyway.
This sounds like a great study.
Here's their methodology.
Unfortunately, I'm not sure that abundance is accurately measured. That would seem to be dependant on the speed of the ships.
Rather the survey seems to be tracking biodiversity - which may explain the lack of clarity in the report.
"As anthropogenic CO2 emissions acidify the oceans..."
They never stop, do they?
Science has been hijacked by activists.
Amongst the wall to wall propaganda on the BBC website, see the article called "What does ocean acidification look like?" (http://www.bbc.co.uk/news/science-environment-34966672).
Mat McGrath and some marine boffin to the tune of The The's "This is the day".
CO2 is driving up temperatures and making oceans all over the world more acidic. Look: This coloured dye how acid something is. Here is some seawater with the dye in it, a nice colour. But when I blow CO2 into it through a straw the colour changes because CO2 is ACID! And look what happens when I put a piece of chalky into vinegar - which is an ACID - it fizzes and bubbles. And these limpet shells are wafer thin because of of corrosive CO2 and I can easily crush them...
And right at the end we get that, actually, the sea won't actually get acidic so don't worry - presumably so the Beeb answer any complaints with "the item did not claim that... etc."
Absolutely shameless they are!
Mardler,
The climate kooks have the answer to the question about why their pH predictions are wrong right in front of them and ignore it.
Heh, so the missing heat may be in the deep after all, silted, not diffused or convected, in the skeletons of algae, representing carbon virtually permanently sequestered from the biosphere.
====================
More Plankton means a less transparent ocean, which means a warmer sea surface (solar photons absorbed in a narrower surface layer). Eventually the sea surface temperature must return to its nominal value, to restore thermal equilibrium. Sea surface temperatures over the last hundred years are not inconsistent with this effect.
coccolithophores are involved in the alkenone proxy, which has been behaving peculiarly in recent samples (which are "too cold"). Maybe this has something to do with CO2 fertilization impacting the proxy.
I don't want to burst any bubbles but this is only likely to be regarded as one of the 'missing' sinks to explain why the Earth consistently gobbles up 60% of manmade CO2. It still leaves the other 40% to cause warming.
"...that means a favourable carbon cycle feedback just got a whole lot bigger.
Excellent news, I'm sure you'll agree."
Actually - no, I don't agree.
I like being warm rather than cold.
I like eating plants that use CO2 to grow.
I like eating some animals that eat plants that ...etc.
A little less CO2 in the atmosphere is not necessarily a good thing.
auralay, the sun and the biome conspire to virtually irreversibly sequester carbon. We did it inadvertently, but the anthropogenic release of sequestered carbon is the greatest thing that has ever happened to the Earth, well, at least the greatest we have ever done.
But the narrative of guilt and fear can't observe or relate that. We can, though.
==================
This claim that CO2 will certainly harm calcifiers is infuriating! In the early 1990s when I was studying Earth Sciences it was almost a throw-away line that increased CO2 dissolved would improve the environment for creatures producing CaCO3 tests, due to the improved availability of carbonate ions. Now I am willing to accept that there is a balance between that and the varying corrosive nature of water with changing pH, but to assume without evidence that more CO2 will make conditions worse is completely unscientific.
It is interesting to note as an aside that nearly all macroscopic carbonate tests (I can't remember about coccoliths) are made from aragonite rather than calcite. Aragonite is metastable in ocean conditions, as opposed to calcite which is stable. This means it dissolves more easily (as there is a lower energy barrier). If dissolution is a serious problem then why would they have evolved to produce a metastable mineral for tests?
Following exhaustive and expensive searching by hundreds/thousands of biologists, none have yet found an organism adversely affected by a slight reduction in ocean alkalinity.
Hundreds/thousands of biologists are thinking of something else to look for. A new career doing something positive for the planet and it's lifeforms?
A few years ago I went to a fascinating seminar at the NOAA Marine Biology lab in Charleston SC. The authors had attempted to chart the "genome of the ocean" at one location nearby. Like me, they expected it to be heinously complex.
But it was worse than they thought: Huge dynamic changes in the absolute and relative populations over periods of hours, days, seasons and years. And that just included the organisms they were able to capture on the record, not the ones currently presenting insurmountable obstacles to DNA sequencing.
I read the abstract's statement "coccolithophore occurrence in the North Atlantic increased from ~2 to over 20%" the same as geoffchambers; that is, an increase of between 2 and 20+ %.
However, it appears that the correct interpretation is that the abundance has increased by an order of magnitude, e.g. here and here.
kim
" but the anthropogenic release of sequestered carbon is the greatest thing that has ever happened to the Earth, well, at least the greatest we have ever done"
I agree completely ^.^ and it is amazing the number of times the biosphere produces an answer just in time to avoid disaster. In the early Devonian period the first trees appeared and for 100 million years trees grew, died, toppled over and were ultimately buried under hundreds of feet of dead and fallen trees. During all of this time the trees were removing CO2 from the atmosphere and levels fell to somewhere close to current levels. The structure of the trees were bound together by a substance called Lignin.
There were no organisms that could break down Lignin and so the CO2 was locked in and our coal fields were created. Towards the end of the 100 million years something called White Rot emerged and this finally enabled Lignin to be broken down and CO2 to be released, phew!
Do we burn enough fossil fuels to keep up with present rates of sequestration? CO2 isn't increasing because of our efforts, or only by a very small amount. Let's hope that the biosphere has some trick up its sleeve as we are heading for 150ppm during the next phase of the Pleistocene. Plants need 150 ppm to grow.
Do we burn enough fossil fuels to keep up with present rates of sequestration? CO2 isn't increasing because of our efforts, or only by a very small amount. Let's hope that the biosphere has some trick up its sleeve as we are heading for 150ppm during the next phase of the Pleistocene. Plants need 150 ppm to grow.
Well relative to warmer climates for the Earth which are more the norm than interglacial climate, the planet is very cold right now and so the Oceans hold a lot of CO2. I guess we need the Milankovitch cycles to take us into a warmer period and release more CO2. I have no idea whether that is on the cards or not hehe.
Tut tut. The university is Johns Hopkins. Calling it John Hopkins is not as bad as calling someone a "looser", or "towing the line"; about on par with "miniscule".
Heh, dung, go long gentian blue.
=========
Dung, 6:29, do we know when the Rot appeared in climate science, that broke down the science and released the idiots into the academic environment?
Excellent news? Well, that depends on whether you like it warmer or colder. We don't have a consensus on that issue yet.
Mikky:
You got the sign wrong: filling the deep blue sea with white cocolithopores increases albedo, thus reflecting more solar energy back into space.
Since this mechanism reduces solar radiative forcing , something must be making up the difference :
Oh no, mister Bishop !,
Your chorus has been underestimating the effect of CO2 again.
Aldus--
Seems the consensus in England is "rather be warmer" judging from the retirement flow to Malta, Gibraltar, Portugal., etc.
Russell, surely that depends on just how deep in the deep blue sea these white cocolithopores are?
Sequestration is possible.
Even sequestered while floating free, deep in the Ocean
"Don't these pesky varmints also increase ocean albedo?" --Green Sand
Plankton concentration is one of the variables considered to have an effect on albedo. My guess is that more little particles (of any sort or color) in the top layer of the ocean would raise absorptivity, lower albedo. There may be a point at which more coccolithophores give no further change in albedo.
One of the things I remember from my Single Honours geology degree at St. Andrews - Cocolith Oforidi .... beautiful sounding name ... totally responsible for the Cretaceous chalk deposits.
I must confess feeling an increasing sense of sadness at the utterly moronic nonsense coming out of Paris. I had never imagined that my generation would see such blatant stupidity. It is truly humbling to realize that for all our technological cleverness we are no more advanced than our ancestors. The ability of humans to abdicate from any sense reason is immense.
By the way - did anyone see Patrick Moore getting 10 mins of prime time on Sky News yesterday. Excellent but surprising.
Sorry Imran- I bow to your recollection of the forams of Fife, but White Cliffs mostly feature E. huxleyi -- a truly ubiquitous chalk former.
MCourtney: Cocoliths need light to photosynthesize, and since half the light is in the first ten meters of water depth, the surface is where you find them. Whlie the deep blue sea has an albedo of about .06-- the same as a maccadam parking lot and just as prone to solar heating . Coccoliths on the other hand are well, white as chalk.
On a watts per square meter basis , bright foram blooms often present larger local radiative forcings than the atmosphere.
'There may be a point at which more coccolithophores give no further change in albedo. '
Dec 2, 2015 at 12:50 AM | jorgekafkazar
It's called paint.
Calcite is calcite and there's lots of it in whitewash and old fashioned cocclit -based chalk white paints
Kim nailed it Green Sand; coccolithophores and other phytoplankton use the light and warmth to multiply and build their little shells. CO2's stability means the little buggers have to use energy to form the larger complexes CACO3
vvussell. once again displays his lack of knowledge regarding things natural or scientific. In vvussell's world the little coccolithophore lie in shining white sheets on the surface of the water.
Remember, the little creature are plants, vvussell!
When biologists and oceanologists describe them as living at the 'surface' they don't mean only in the water tension film.
Imagine that? And that's using the small estimates. Increase by a factor of 10 and that is 15 million tons (14 billion kilograms) of calcite.
Add a lot of pressure and some local magmatic heating, over time of course, and that calcite can eventually be turned into marble. The purer the layers of calcite builder's shells are, eventually becoming marble, the whiter the marble will be. Shades of Michelangelo!
Could we carve some fitting words on the marble for you? Perhaps?
"CAGW is a farce,
so much money is waste,
like the life you've spent,
pulling commentary out your arse."
I refer the right honorable ignoramus from the state of a the k to the remarks made on coccolith photosynthesis and the dimensions of the euphotic zone one hour before he started raving.
Vide supra
For literally dozens of satellite images of dense turquose coccolith blooms, see:
https://www.google.com/search?q=coccolithophore+blooms+are+visible+from+space+because&biw=1660&bih=901&source=lnms&tbm=isch&sa=X&ved=0ahUKEwjlppLrtrzJAhUK4iYKHQKIDt0Q_AUIBigB
Next !
Or just google :
coccolithophore blooms are visible from space
and hit IMAGES
auralay;
Right. Reducing atmospheric CO2 is hardly "favourable" from the POV of the plankton et al.
vvussell:
You mean like the brilliant statement left by one vvussell about calcite being white and in paints... Actually it is in a whole sea of products from paints and putties through paper. Though titanium dioxide has mostly replaced powdered calcite in paints and paper.
I assume that your comment
is now retracted" And you can specify the albedo reduction explicitly AND identify how much energy the coccolithophores consume in constructing their shells?
Larger local 'radiative forcings'... Sound almost important. Exactly how much of a forcing change?
Don't forget that this ignoramus pointed out what the blooms look like. Which probably caused you to 'look' up coccolithophore blooms. I'm glad you learned something!
My apologies Doubting Rich, I should've commented on this earlier.
Yes and no.
Aragonite was theorized to be more susceptible to the acidic influences of dissolved carbon dioxide though the rationale was never explained successfully.
Part of that confusion was because of how little aragonite was found some phytoplankton skeletons and yes, our little friend 'Emiliania huxleyi' coccolithophore's skeletons were thought to suffer dissolution of their shells in the winter.
Aragonite and calcite are both CaCO3, they differ in crystal form which also means they differ in optical properties and x-ray diffraction properties. Which is a good thing as that is often the only method to truly determine which one is present.
Both aragonite and calcite are considered polymorphs of the other and both minerals form crystal structures 'after' the form of the original structure so that calcite can be found as a follower after aragonite or aragonite can be found as a follower after calcite.
coccolithophores prefer constructing calcite skeletons; aragonite presence is not common.
Where aragonite is more common are in bivalves, (clams, oysters, mussels, etc.). The iridescence that gives pearls their luster are layers of aragonite.
Both calcite and aragonite are transparent and translucent in their base states. Rather necessary for that light to penetrate into where the chlorophyll is.
I'm OK with AK persisting in his delusional views; those who do not share them will find a pretty picture of a high albedo coccolith bloom has long featued in the logo of this blog:
http://vvattsupwiththat.blogspot.com/2015/12/cop-21-laki-horror-show.html
Those actually curious about radiative forcing by bright hydrosol and coccolith dispersions will find a peer-reviewed discussion of the underlying Mie theory here:
http://link.springer.com/article/10.1007%2Fs10584-010-9965-8
Cue splutterings about how much Watts doesn't like the published science.
Overview of attention for article published in Climatic Change, December 2010
That puts the score at Twitter Attention 1, Scientific Journal Citations 34 of which Springer lists
Advances in Colloid and Interface Science
Advances in Water Resources
Annual Review of Earth and Planetary Science
Earth's Future
Economics Research International
Economics Research International
Environmental Research Letters
Environmental Science & Technology
Geophysical Research Letters
Journal of Geophysical Research: Atmospheres
Philosophical Transactions of the Royal Society
Royal Society of Chemistry Advances
Book Chapter:
Engineering Response to Climate Change, Second Edition
If you try harder, I'm sure you can find a search engine that hasn't discovered either, but what's the point ?